Targeting Thymic Stromal Lymphopoietin
TSLP is one of the key drivers of the asthmatic pathophysiology as it is produced by the airway epithelium in response to inhaled allergens and proinflammatory stressors. Targeting TSLP may be interesting because of its upstream role in the asthma cascade .
Tezepelumab
Tezepelumab binds to TSLP, inhibiting its stimulating activity on dendritic cells and innate lymphoid cells thus preventing the induction of type 2 cytokines . It is administered SC. It has been investigated by a phase II trial in patients with uncontrolled asthma and multiple exacerbations in the previous year despite receiving medium to high dose ICS . The exacerbation risk was significantly reduced in tezepelumab groups – irrespective of the baseline blood eosinophil count – compared to placebo by 62% in the low-dose group, 71% in the medium-dose group and 66% in the high-dose group.
Daclizumab
Network meta-analysis
Nih Launches Trial Of Monoclonal Antibody To Treat Asthma In Urban Youth
Study also will examine relationship of airway gene network activity to health outcomes.
The National Institutes of Health has launched a clinical trial testing whether a monoclonal antibody, dupilumab, can reduce asthma attacks and improve lung function and asthma symptoms in children with poorly controlled allergic asthma who live in low-income urban neighborhoods. The investigators also aim to define the activity levels of asthma-associated gene networks that correspond to specific health outcomes during antibody treatment in these children, most of whom are anticipated to be Black or Hispanic. The National Institute of Allergy and Infectious Diseases , part of NIH, is sponsoring and co-funding the trial, called Prevention of Asthma Exacerbations Using Dupilumab in Urban Children and Adolescents, or PANDA.
Dupilumab is approved by the U.S. Food and Drug Administration as an add-on maintenance treatment for certain types of moderate-to-severe asthma in people ages 6 years and older. However, little data exist on the effectiveness of the drug in Black and Hispanic children, even though severe asthma disproportionately affects U.S. children in these racial and ethnic groups. The new NIAID study will help fill this knowledge gap.
More information about the PANDA trial, including study site locations and contacts, is available in ClinicalTrials.gov under study identifier NCT05347771.
NIHTurning Discovery Into Health®
How Do Monoclonal Antibodies Help Severe Asthma
Biologic therapies are ground-breaking as they target pathways in the immune system that cause the problematic airway inflammation seen in asthma. They are indicated for use in severe eosinophilic asthma and severe allergic asthma.
Professor Peter Gibson says, biologics therapies make a big difference, for example, a 50 per cent reduction in the rate of severe attacks. We expect to see reduced presentations for asthma attacks in severe asthma patients.
Hear from someonereceiving Biologics and learn more about severe asthma and Biologicshere.
Read Also: How To Improve Asthma Naturally
Stay Up To Date On Covid
COVID-19 vaccines are effective at protecting peopleespecially those who are up to date from getting seriously ill, being hospitalized, and even dying. As with vaccines for other diseases, you are protected best when you stay up to date with your COVID-19 vaccines. The people you live or spend time with can help protect you and themselves by staying up to date on their COVID-19 vaccines too.
You are up to date with your COVID-19 vaccines when you have received all doses in the primary series and all boosters recommended for you, when eligible. Since your immune response to COVID-19 vaccination may not be as strong as in people who are not immunocompromised, you have different recommendations for COVID-19 vaccines, including boosters. Learn more about COVID-19 vaccine recommendations for people who are moderately or severely immunocompromised.
To find COVID-19 vaccine locations near you: Search vaccines.gov, text your ZIP code to 438829, or call 1-800-232-0233.
Monoclonal Antibodies Anaphylaxis Risk For Severe Asthma Patients
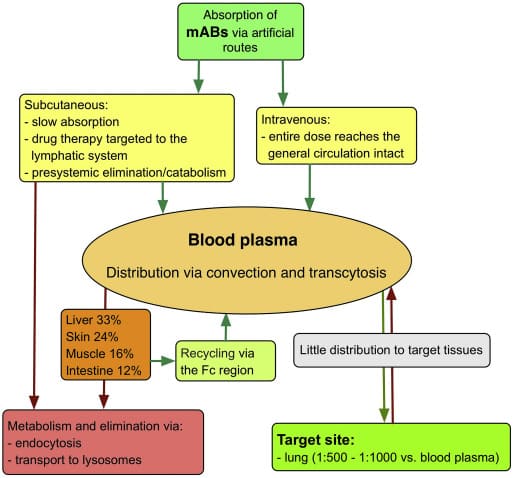
Most monoclonal antibodies increase the risk of anaphylaxis in severe asthma patients, according to a report published in Clinical and Translational Allergy. The study found 4 out of 5 common mAbs were associated with an increased risk of anaphylaxis. Each of the therapies had a different risk profile. One monoclonal antibody dupilumab exhibited a very low risk. The patients in this study were mainly young and middle-aged adults.
You May Like: How To Manage Asthma At Home
What Conditions Are Monoclonal Antibodies Used To Treat
Monoclonal antibody therapy is used to treat a wide range of conditions, depending on the antigen it is targeting. These conditions include:
- Many types of cancers including breast cancer, leukemia, lymphoma, and cervical cancer, among others
- Autoimmune disorders, such as rheumatoid arthritis, multiple sclerosis, and lupus
- Organ transplant rejection
- Infections, such as Covid-19
- Asthma, by preventing allergic reactions to the allergens
Note that indications may vary amongst products so speak with your healthcare provider for medical advice on which drug will work best for your medical condition.
List Of Therapeutic Monoclonal Antibodies
This is a list of therapeutic, diagnostic and preventive monoclonal antibodies, antibodies that are clones of a single parent cell. When used as drugs, the International Nonproprietary Names end in -mab. The remaining syllables of the INNs, as well as the column , are explained in Nomenclature of monoclonal antibodies.
The abbreviations in the column Type are as follows:
- mab: whole monoclonal antibody
- Fab: fragment, antigen-binding
- F2: fragment, antigen-binding, including hinge region
- Fab’: fragment, antigen-binding, including hinge region
This list of over 500 monoclonal antibodies includes approved and investigational drugs as well as drugs that have been withdrawn from market consequently, the column Use does not necessarily indicate clinical usage. See the list of FDA-approved therapeutic monoclonal antibodies in the monoclonal antibody therapy page.
Read Also: Is Asthma A Autoimmune Disease
How Do You Take Monoclonal Antibodies
Biologic therapies are delivered by injection every 2 to 8 weeks.
After treatment is started by a specialist, your ongoing doses can be given by your GP or Nurse. Some biologics are available as a pre-filled syringe for you to give yourself at home.
Access the Severe Asthma and COVID-19 resource for consumers and health professionalshere.
How Do People Get Access To Biologic Treatments
Biologic treatments for asthma are only available to people who have been diagnosed with severe asthma. You need to be referred to a severe asthma service before you can be considered for one.
Your specialist will only recommend biologic treatment if you meet the necessary criteria for accessing these treatments and if youve been taking all your medicines as prescribed.
To start the assessment for biologic treatments youll need an appointment at a specialist asthma clinic. This is for tests to assess:
- how far youve been able to stick to your medicine routine
- what type of severe asthma you have.
A specialist team will talk through your test results and discuss the most appropriate biologic for you to try.
Individual biologic treatments have their own criteria for who can access them too.
If youre eligible for more than one biologic you should be offered a choice, and your specialist should involve you in deciding which one to try.
How soon can you start using biologics?
Its not always easy to access biologic treatments. On average it takes about a year from being referred to a severe asthma service to getting a biologic. But that can vary.
A lot depends on how well youve been sticking to your current medicines, any other conditions you have, and how well matched you are to a biologic treatment .
Also Check: Doctors Who Treat Asthma Are Called
Possible Side Effects Of Monoclonal Antibodies
Monoclonal antibodies are given intravenously . The antibodies themselves are proteins, so giving them can sometimes cause something like an allergic reaction. This is more common while the drug is first being given. Possible side effects can include:
- Low blood pressure
Compared with chemotherapy drugs, naked mAbs tend to have fewer serious side effects. But they can still cause problems in some people. Some mAbs can have side effects that are related to the antigens they target. For example:
- Bevacizumab is an mAb that targets a protein called VEGF that affects tumor blood vessel growth. It can cause side effects such as high blood pressure, bleeding, poor wound healing, blood clots, and kidney damage.
- Cetuximab is an antibody that targets a cell protein called EGFR, which is found on normal skin cells . This drug can cause serious rashes in some people.
Fda Approves Tezspire In The Us For Severe Asthma
First and Only Biologic to Consistently and Significantly Reduce Exacerbations in a Broad Population of Severe Asthma PatientsOnly Biologic for Severe Asthma Approved With no Phenotype or Biomarker Limitations
THOUSAND OAKS, Calif. today announced that the U.S. Food and Drug Administration has approved Amgen and AstraZeneca’s Tezspire for the add-on maintenance treatment of adult and pediatric patients aged 12 years and older with severe asthma.1
To view the Multimedia News Release, please visit:
Tezspire was approved following a Priority Review by the FDA and based on results from the PATHFINDER clinical trial program. The application included results from the pivotal NAVIGATOR Phase 3 trial in which Tezspire demonstrated superiority across every primary and key secondary endpoint in patients with severe asthma, compared to placebo, when added to standard therapy.2
Results from the NAVIGATOR Phase 3 trial were published in in May 2021 .2 In clinical studies of Tezspire, the most common adverse reactions were nasopharyngitis, upper respiratory tract infection and headache.2
Tezspire is under regulatory review in the EU, Japan and several other countries around the world.
U.S. IndicationTezspire is a first-in-class medicine indicated for the add-on maintenance treatment of adult and pediatric patients aged 12 years and older with severe asthma.
CONTACT:
Also Check: What Are The Symptoms Of Allergy Induced Asthma
What Mabs Are Made Of
Monoclonal antibodies are man-made proteins that act like human antibodies in the immune system. There are 4 different ways they can be made and are named based on what they are made of.
- Murine: These are made from mouse proteins and the names of the treatments end in -omab.
- Chimeric: These proteins are a combination of part mouse and part human and the names of the treatments end in -ximab.
- Humanized: These are made from small parts of mouse proteins attached to human proteins and the names of the treatments end in -zumab
- Human: These are fully human proteins and the names of the treatments end in -umab.
What Are Monoclonal Antibodies

Your immune system creates antibodies against dangerous invaders. If you have an autoimmune disorder, those antibodies may attack healthy parts of your body.
cascade , as it has many parts.
The cascade includes some factors that increase inflammation and some that decrease it. In healthy people, those factors are in balance. If you have an immune-mediated disorder, they are often out of balance.
Among those factors are different proteins made by white blood cells. Monoclonal antibodies each target a specific protein, aiming to keep the factors in balance. If the drug targets the right part of the cascade, it should reduce symptoms in people with severe asthma.
Currently, six monoclonal antibodies are Food and Drug Administration -approved to treat severe asthma. Lets take a look at them.
Omalizumab binds to Immunoglobulin E , an antibody you produce that is involved in allergic reactions. This mAb is for people with severe allergic asthma caused by exposure to allergens like dust mites, pollen, and dander.
Some of the possible side effects of this medication include:
- swelling or itching at the injection site
FDA approval . It works by targeting thymic stromal lymphopoietin , which is a component of airway inflammation.
Tezepelumab is for use with all forms of severe asthma. It is the first biologic not limited to a subtype of asthma.
You usually receive a dose once every 4 weeks. A healthcare professional administers it in a clinical setting.
- soreness at the injection site
Recommended Reading: What Is Acute Exacerbation Of Bronchial Asthma
How Much Do Monoclonal Antibodies Cost
Monoclonal antibodies are very expensive with an average cost of around $100,000 per year. The mAbs used for cancer can be even more than the average while other conditions can be lower, but still cost about $20,000 per year.
You can purchase monoclonal antibodies for $49 per month from NiceRx if eligible for assistance. Prices at the pharmacy vary by your location, strength, and quantity, as well as your insurance status.
Therapeutic Monoclonal Antibodies Approved Or In Review In The Eu Or Us
Table notes: *, Country-specific approval. #, Withdrawn or marketing discontinued for the first approved indication. NA, not approved or in review in the EU not approved or information on review status not available in US.
Additional notes:
1. Of the antibody therapeutics listed in the table, the following products were not first approved in the EU or US:Satralizumab , first approved in Canada in May 2020 Risankizumab, first approved in Japan in March 2019 Romosozumab, first approved in Japan on January 8, 2019 Sarilumab, first approved in Canada on January 12, 2017 Brodalumab, first approved in Japan on July 4, 2016 Secukinumab, first approved in Japan on December 26, 2014 Mogamulizumab, first approved in Japan on March 30, 2012 Tocilizumab, first approved in Japan on April 11, 2005 Cetuximab, first approved in Switzerland on December 1, 2003.
2. Several antibody therapeutics are approved for marketing in regions other than the EU or US. These products include:Nimotuzumab , humanized anti-EGFR IgG1 approved in numerous countries for various forms of solid tumors starting in the 2000s.
Itolizumab , humanized anti-CD6 IgG1 approved in India in January 2013 for psoriasis.RabiMabs , mixture of 2 anti-rabies virus mAbs approved in India in 2019Rmab , human anti-rabies virus G glycoprotein IgG1 approved in India in 2016 for post-exposure prophylaxis of rabies.
Casirivimab and imdevimab , anti-SARS-CoV-2 antibody approved in Australia in Oct 2021.
References:
Don’t Miss: Can Cold Air Trigger An Asthma Attack
Learn How To Get Treatment Quickly
If you test positive for COVID-19, oral antiviral and monoclonal antibody treatments are available for people who are more likely to get very sick. Learn more about COVID-19 treatment.
Dont delay: Treatment must be started right away to be effective. Talk to your healthcare provider about what treatment options are best for you.
Antibody Therapeutics Approved Or In Regulatory Review In The Eu Or Us
The Antibody Society maintains a comprehensive list of approved antibody therapeutics and those in regulatory review in the European Union or United States . In the table below, product candidates undergoing review are listed first, and approved products are listed in reverse chronological order by year of first approval. Products that were granted approvals but subsequently withdrawn from the market are included in the table. Biosimilar products are excluded.
- European Medicines Agencys medicine-related data can be , and information about medicines under evaluation can be found here.
- Information about therapeutics approved in the US can be found at Drugs@FDA.
- Antibodies to watch in 2022. includes details about antibody therapeutics that may enter regulatory review or be granted approvals in 2022.
- Antibody therapeutics that were granted a first approval in regions other than the EU or US are listed at the end of the table below.
As of Oct 28, 2022, 19 investigational antibody therapeutics are in regulatory review in either the US or EU.
During January 1 Oct 28, 2022, 9 antibody therapeutics were granted first approvals in either the US or EU.
Don’t Miss: What Causes Someone To Get Asthma
Risks And Side Effects Of Biologics
As with all medicines, biologic treatments can have risks and side effects.
Common side effects
The patient information leaflet for your biologic treatment will list all the possible risks and side effects. Read the leaflet carefully before starting your treatment.
Some common side effects include:
- soreness at the injection site .
Always talk to your doctor or specialist team if youre worried about any of these risks or side effects.
Severe allergic reactions
Biologic treatments for asthma have an increased risk of a severe allergic reaction . The risk is rare and more likely if you have a history of allergic reactions or anaphylaxis.
You may be asked to stay in the clinic for a short time after your first few treatments, so your healthcare team can make sure youre not at risk of an extreme allergic reaction.
If youve been told you can self-treat at home, its important to know the signs of an allergic reaction, such as difficulty breathing, swelling, or feeling dizzy or unwell.
Parasitic infections
Taking biologics can, rarely, make you less resistant to parasitic infections such as cyclospora, intestinal worms, or malaria.
This means your body cannot fight off infections like this so well. Get advice from your doctor if youre travelling to regions in the world where parasitic infections are more common than they are in the UK.
How Do Monoclonal Antibodies Work
Monoclonal antibodies affect the immune system to function in a variety of ways. Some examples may include:
- Flags cancer cells so the immune systems cells can locate and destroy the targeted cells.
- Directly attacks cancer cells, causing them to self-destruct.
- Destroys the cell membrane of cancer cells.
- Blocks cell growth.
- Delivers radiation and other cancer treatments which can help minimize the radiation effect on healthy cells.
- Blocks inhibitors in the immune system to improve the bodys natural response to cancer cells.
- Blocks a virus from attaching to cells.
Read Also: What To Do When Having An Asthma Attack
