Asthma Medication Not Working Try Another
People who supplement rescue inhalers with a second asthma medication sometimes get little relief at first, but theres good news: Those who keep trying different options often find a medication that works, according to Rutgers researchers.
The researchers analyzed data from 2,025 patients who used any of six FDA-approved severe asthma treatments known as biologics because they contain monoclonal antibodies found in living organisms.
The practical takeaway here is pretty simple: Patients who arent getting good relief from a particular biologic should try others, said lead author Reynold A. Panettieri Jr., vice chancellor for translational medicine and science director at the Rutgers Institute for Translational Medicine.
Overall, according to findings published in the Annals of Allergy, Asthma & Immunology, these injectable medications provided significant relief. Patients starting biologics during the study period experienced a 58 percent reduction in exacerbations, and 89 percent of those who used biologics at any point during the study period continued to do so at the end.
Some 324 patients switched medications at least once during the study period most commonly because their symptoms worsened on the first medication or because initially strong effects waned over time and their strategy was rewarded, the researchers found. Switching medications was consistently associated with a reduction in exacerbations.
Biologics For Asthma: Fixing The Future
Open the medicine cabinet in Ellies bathroom and you are likely to see a lot of asthma medicine. Long term asthma control meds, quick-relief asthma rescue meds, youll see allergy-medicines but you wont see any biologics for asthma. She cant afford those.
Ellie is a mother of two in New York State. She is married and works full time. Ellie has been managing with multiple drugs for years because she was diagnosed with asthma as a child. I talked to my doctor about going onto a biologic drug that I had done some research on. Biologics supposedly fix the things that cause asthma flares in the first place. I get flares a lot because my job is really stressful . My doctor thought it was worth a try so he prescribed it but my insurance refused it. They said it was experimental.
Nucala Is The First Biologic Approved In The Us For Six To 11
Issued: London UK
GlaxoSmithKline today announced that the US Food and Drug Administration has approved Nucala for use in children as young as six years old who are living with severe eosinophilic asthma. Nucala is the only targeted biologic to be approved for the condition in the six to 11-year age group in the US.
Dr Hal Barron, Chief Scientific Officer and President, R& D, GSK, said: Children with severe eosinophilic asthma currently have limited treatment choices available to them. We believe this important new indication for Nucala is a significant development for these children and their families.
Tonya Winders, CEO and President, Allergy and Asthma Network, noted: As a mother of children who suffer from asthma, I know first-hand the huge impact it has on a family, from the constant worry about your child being hospitalised, to practical issues like arranging time off work to care for them. Having Nucala approved as the first biologic for treating severe eosinophilic asthma in this young age group represents a significant step forward for the asthma community.
Dr Daniel Jackson, MD, Department of Pediatrics, University of Wisconsin, added: Severe eosinophilic asthma in children is a complicated condition that can be extremely challenging to treat. Nucala has made a difference for many adults and adolescents living with severe asthma. This approval is an important development, giving physicians like me a much-needed option to consider for our paediatric patients.
Read Also: When To See A Doctor For Asthma Cough
When Will Tezspire Be Available
In January 2022, AstraZeneca announced that Tezspire is ready for distribution in the U.S. So you should be able to access it soon if your healthcare provider recommends it for you.
If you have severe asthma and youre interested in Tezspire, talk with your healthcare provider. They can tell you more about this new medication and if you should get it or not.
How Effective Is Tezspire
There are several ways to determine the effectiveness of an asthma medication. Experts generally consider a medication to be effective if it controls airway swelling. Less swollen airways help improve asthma symptoms and lower the chances of having an asthma attack.
In a key study that led to Tezspires FDA approval, researchers found that people with severe asthma can benefit from Tezspire. This was regardless of the cause of a person’s asthma. In fact, in the study, it lowered the risk of asthma attacks by more than 70%.
Read Also: Can Asthma Make You Tired
Biologics May Be The Answer For The Asthma Problem
It is going to take some time before biologics become more widely used. Biologics are out of reach for most patients due to their cost. For those with severe asthma, the improvement to quality of life and reduction in flare ups make them worthwhile option. When deciding how accessible biologics will be the long-term reduction in the economic burden of asthma must be taken into consideration. The Asthma and Allergy Foundation of America says that for now biologics are not well known to most asthma patients. Yet, those who have used them found them to be life altering, especially on quality of life issues.
For now, biologics are beyond the reach of most. Kenneth Mendez, the President of AAFA sums up the paradox well. Despite the promise of biologics, if they are cost-prohibitive for the majority of the population, their overall impact will be minimal. In other words, if you cant afford them, you cant use them. And if that is the case, we can expect the economic impact of asthma to rise as patients run into more barriers to effective treatment.
Biologics And Allergic Asthma
If allergic asthma symptoms are constantly disrupting your daily life, even though you use control medications like inhalers, it may be time to add on a biologic.
Biologics are medications you take as a shot or IV infusion. Theyâre monoclonal antibodies, which are human-made blood proteins. Scientists make them using cells from living organisms. Biologics bind to parts of your immune system that are responsible for asthmatic inflammation and turn it down.
âBiologics suppress this specific targeted immune response so that the asthma can be controlled without needing a broader immunosuppressant like prednisone, which comes with many side effects,â says Purvi Parikh, MD, allergist and immunologist with Allergy & Asthma Network.
Biologics are meant to be an add-on to other types of allergic asthma treatments, not a standalone medication.
These may include:
Immunotherapy. This therapy involves seeing an allergist for allergy shots. The shots contain very small doses of the allergen that triggers a reaction in you. Over time, your body may become less reactive to the allergen.
Allergy medications. Although they arenât treatments for asthma itself, oral and nasal antihistamines and decongestants, as well as corticosteroid and cromolyn nasal sprays, can help ease the allergic reaction causing your asthma symptoms.
Don’t Miss: How Many People Have Asthma
Fda Approves Tezspire In The Us For Severe Asthma
First and Only Biologic to Consistently and Significantly Reduce Exacerbations in a Broad Population of Severe Asthma PatientsOnly Biologic for Severe Asthma Approved With no Phenotype or Biomarker Limitations
THOUSAND OAKS, Calif. today announced that the U.S. Food and Drug Administration has approved Amgen and AstraZeneca’s Tezspire for the add-on maintenance treatment of adult and pediatric patients aged 12 years and older with severe asthma.1
To view the Multimedia News Release, please visit:
Tezspire was approved following a Priority Review by the FDA and based on results from the PATHFINDER clinical trial program. The application included results from the pivotal NAVIGATOR Phase 3 trial in which Tezspire demonstrated superiority across every primary and key secondary endpoint in patients with severe asthma, compared to placebo, when added to standard therapy.2
Results from the NAVIGATOR Phase 3 trial were published in in May 2021 .2 In clinical studies of Tezspire, the most common adverse reactions were nasopharyngitis, upper respiratory tract infection and headache.2
Tezspire is under regulatory review in the EU, Japan and several other countries around the world.
U.S. IndicationTezspire is a first-in-class medicine indicated for the add-on maintenance treatment of adult and pediatric patients aged 12 years and older with severe asthma.
CONTACT:
How Does Biologic Therapy Treat Severe Asthma
Asthma is a complex disease. Many patients are able to control asthma with inhaler therapy. But some patients with severe asthma have episodes of frequent symptoms and episodes where the asthma gets much worse even when they use more than one inhaler. Now there are new options called biologic therapy to help patients with severe asthma. Biologic therapies offer new ways of treatment because they target different molecules in the body that contribute to asthma.
Also Check: Arizona Asthma And Allergy Institute Reviews
A Surge Of Biologics For Severe Asthma
Rosanna Sutherby, Pharm.D.MHE Publication
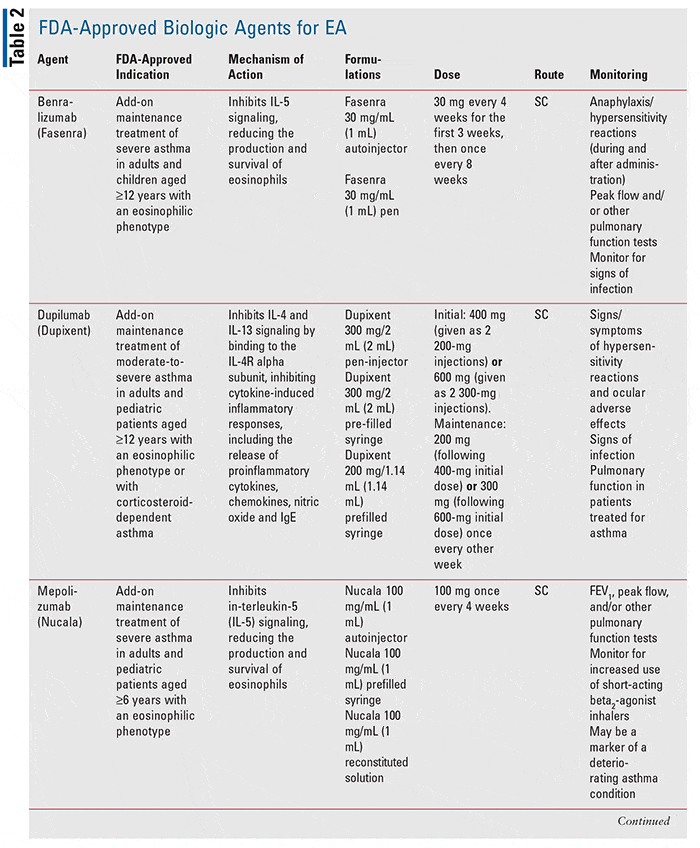
Six biologics are on the market and more are in the pipeline. Biologics targetthe inflammatory cytokines involved in the pathogenesis of asthma.
Asthma is a chronic respiratory disease that causes narrowing and inflammation of the airways that compromises breathing and, in some cases, can be fatal. Symptoms include coughing, wheezing, shortness of breath, chest tightness and chest pain. Nearly 25 million people in the United States have asthma, and 10 people die from the disease daily.
Current asthma treatment aims to prevent exacerbations and relieve symptoms when exacerbations occur. Traditional therapies include inhaled corticosteroids, long- and short-acting beta-agonist inhalers, long- and short-acting inhaled anticholinergics, leukotriene receptor antagonists and oral corticosteroids.
Most asthma cases are treatable using the currently available therapies mentioned. But about 10% of people with asthma do not respond to standard treatments and are considered to have severe asthma. The Global Initiative for Asthma defines severe asthma as asthma that is not controlled by proper use of high-dose inhaled corticosteroids and long-acting beta-agonists or that worsens when high doses of these drugs are lowered.
Severe asthma is classified into three types based on patients response to treatment and the presence of certain biomarkers: allergic asthma, eosinophilic asthma, and noneosinophilic asthma.
How Is A Biologic Different From Other Drugs
The term biologics covers a wide selection of drug types. According to the FDA Biological products include a wide range of products such as vaccines, blood, blood components, allergenics, certain cells, gene therapy and other tissues. Biologics are grown in live organisms which makes them different from most drugs. This makes them fragile and at risk for damage from temperature, spoilage or contamination. In contrast, most manufactured drugs, the type you see in pill form, are made in a lab setting and do not use live biological material.
You May Like: Can Third Hand Smoke Cause Asthma
Fda Approves Tezepelumab For Severe Asthma
The FDA Friday approved tezepelumab, the first biologic that targets thymic stromal lymphopoietin , for severe asthma.
Levels of TSLP, an epithelial cytokine, are linked with disease severity, airway obstruction, and resistance to glucocorticoids. The biologic, to be sold as Tezspire by Amgen and AstraZeneca, was approved as an add-on maintenance treatment of adult and pediatric patients aged 12 years and older.
Results of the drug, published last spring, showed that it cut exacerbations by 56%. It will become the first and only biologic for severe asthma that is not labeled for a specific phenotypeeosinophilic or allergicor biomarker.
On Thursday, the Institute for Clinical and Economic Review released its final report assessing the comparative clinical effectiveness and value of tezepelumab. ICER said its health-benefit price benchmark range for the drug is between $9,000-$12,100 per year.
Tezepelumab was approved following a Priority Review by the FDA and based on results from the PATHFINDER clinical trial program. The application included results from the pivotal NAVIGATOR phase 3 trial in which the drug demonstrated superiority across every primary and key secondary endpoint in patients with severe asthma, compared with placebo, when added to standard therapy.
The most common adverse reactions were nasopharyngitis, upper respiratory tract infection and headache.
What Do Biologics Do
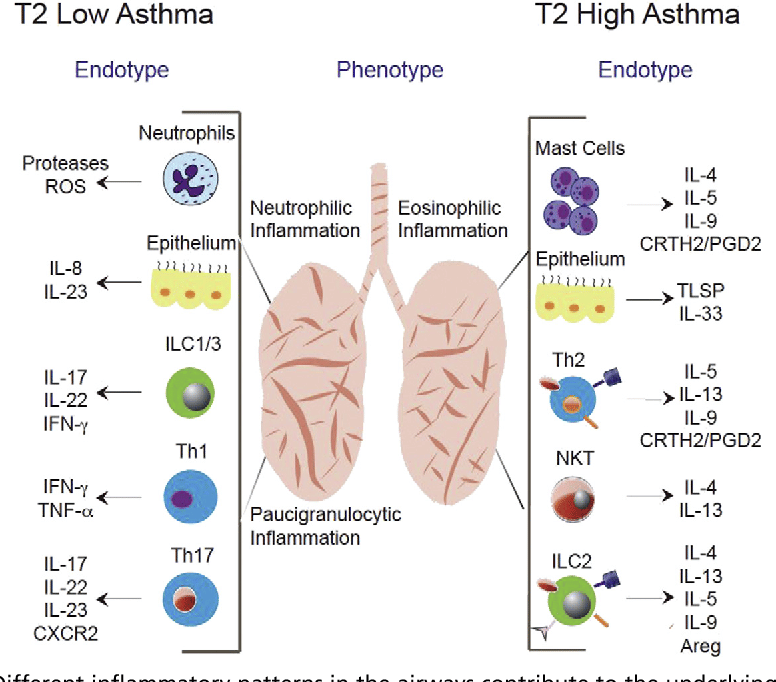
Asthma is an abnormal immune response. During this response, immune cells recognize harmless proteins you inhale as harmful. One example of a harmless protein is allergens, like dust mites.
Many asthma subgroups are regulated by a type of white blood cell called T-helper 2 cells. They are told to release chemicals that encourage the production of antibodies. These antibodies are called dust mite IgE antibodies. This begins a series of chemical reactions that lead to the release of other chemicals such as IL13 and IL5.3
So, biologics block the effects of these chemicals. In turn, they suppress the abnormal immune response responsible for asthma. They down-regulate this immune response. So, in this way, biologics block the specific processes that cause asthma, as opposed to just treating symptoms.
Also Check: How To Deal With Asthma At Night
What Is A Biosimilar
You may have heard the term Biosimilar. A biosimilar is a biological product that is highly similar to and has no clinically meaningful differences from an existing FDA-approved biologic. Biosimilars are kind of like generic drugs except of course they are similar to biologics so are grown using live cells just like biologics. The Aimed Alliance reported that biosimilars will be paid for under Medicare Part B in the same way Generic drugs would be.
Nucala Cinqair And Fasenra
Nucala, Cinqair, and Fasenra target disease-fighting white blood cells that are involved in allergic inflammation. These cells are called eosinophils.
Eosinophils are the predominant cells that play a role in the inflammatory response in the lungs of asthmatics.
If a person has a large number of eosinophils, they may have eosinophilic asthma. In this case, their eosinophils can cause inflammation in the airways and respiratory system, producing asthma symptoms.
Nucala, Cinqair, and Fasenra can bind to these eosinophils to prevent them from triggering an immune response. By doing this, these medications can reduce a persons asthma symptoms.
Medical professionals also use Nucala to treat eosinophilic granulomatosis with polyangiitis, formerly known as Churg Strauss Syndrome. This is a rare small vessel vasculitis, or inflammation of blood vessels, strongly associated with asthma.
You May Like: Where To Recycle Asthma Inhalers
Looking To The Future
While tezepelumab is the first biologic targeting TSLP to be approved by the FDA, there are additional biologics in the pipeline. Another anti-TSLP treatment under study is CSJ117, a fully human neutralizing antibody antigen-binding fragment from Novartis. Unlike the subcutaneously injectable tezepelumab, CSJ117 is being created as an inhaled formulation, possibly offering greater convenience to patients.ix Additional new biologics that dont target TSLP are also undergoing testing. For example, a phase IIb clinical trial of Roches MSTT1041A, an anti-ST2 human MAB was recently completed but no results have been published. Other possible future treatments for severe asthma could target the kinases and PGD2 .x Undoubtedly, many more innovative biologic treatments for severe asthma will be developed in the future.
Fda Approves Tezspire Biologic To Treat Severe Asthma For People Age 12 And Older
The Asthma and Allergy Foundation of America is sharing this press release from Amgen to bring you the latest research news.
First and Only Biologic to Consistently and Significantly Reduce Exacerbations in a Broad Population of Severe Asthma Patients
Only Biologic for Severe Asthma Approved With no Phenotype or Biomarker Limitations
THOUSAND OAKS, Calif. Amgen today announced that the U.S. Food and Drug Administration has approved Amgen and AstraZenecas Tezspire for the add-on maintenance treatment of adult and pediatric patients aged 12 years and older with severe asthma.1
Tezspire was approved following a Priority Review by the FDA and based on results from the PATHFINDER clinical trial program. The application included results from the pivotal NAVIGATOR Phase 3 trial in which Tezspire demonstrated superiority across every primary and key secondary endpoint in patients with severe asthma, compared to placebo, when added to standard therapy.2
Results from the NAVIGATOR Phase 3 trial were published in The New England Journal of Medicine in May 2021.2 In clinical studies of Tezspire, the most common adverse reactions were nasopharyngitis, upper respiratory tract infection and headache.2
Tezspire is under regulatory review in the EU, Japan and several other countries around the world.
Tezspire U.S. IndicationTezspire is a first-in-class medicine indicated for the add-on maintenance treatment of adult and pediatric patients aged 12 years and older with severe asthma.
Don’t Miss: Good Breathing Exercises For Asthma
Role Of The Pharmacist
Pharmacists play a critical role in the selection of treatment for EA, as well as the education and assessment of patients with EA. Although the benefits and risks of biologic therapy for EA must be carefully weighed, other patient factors must also be carefully considered, such as affordability, access, and preference. Pharmacists should also assess adherence to ICS treatment and rule out other causes of asthma exacerbation prior to adjunctive treatment recommendations for severe EA. In addition, pharmacists can assist patients with referrals to support services as well as psychological services to manage emotional, social, and other burdens of EA and its management.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
Biologics And Small Molecules Under Investigation
IL-25 and IL-33 hold potential as upstream targets for the treatment of asthma. Although no biologics under investigation directly target IL-25, two anti-IL-33 antibodies, i.e., REGN3500 and ANB020 or etokimab, are under investigation .
Various small molecules targeting specific inflammatory pathways are also being evaluated . The chemoattractant receptor-homologous molecule expressed on Th2 cells , which binds to PGD2 , is one potential key target. CRTH2, expressed on eosinophils, mast cells, and basophils, and PGD2 are involved in allergic inflammation .
Other promising targets are the STAT5/6 Src homology 2 domains. In mouse models, PM-43Ia small-molecule inhibitor of the STAT6 Src homology 2 domain that prevents recruitment to the IL-4R docking site and phosphorylation of Tyr641potently inhibited STAT5- and STAT6-dependent allergic airway disease and reversed preexisting allergic airway disease .
Overall, these targeted therapies may play a role in individualized treatment of severe asthma however, their application will likely be limited to patients with certain phenotypes who meet the specific criteria for use.
Recommended Reading: How To Cure Asthma Forever
