Of Participation And Request For Credit
Fees for participating and receiving CME credit for this activity are as posted on The ObG Project website. During the period from 03-23-2020 through 03-21-2021, participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please complete the post-test and evaluation. Upon registering and successfully completing the post-test with a score of 100% and the activity evaluation, your certificate will be made available immediately.
For Pharmacists: Upon successfully completing the post-test with a score of 100% and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Black Box Warning For Singulair
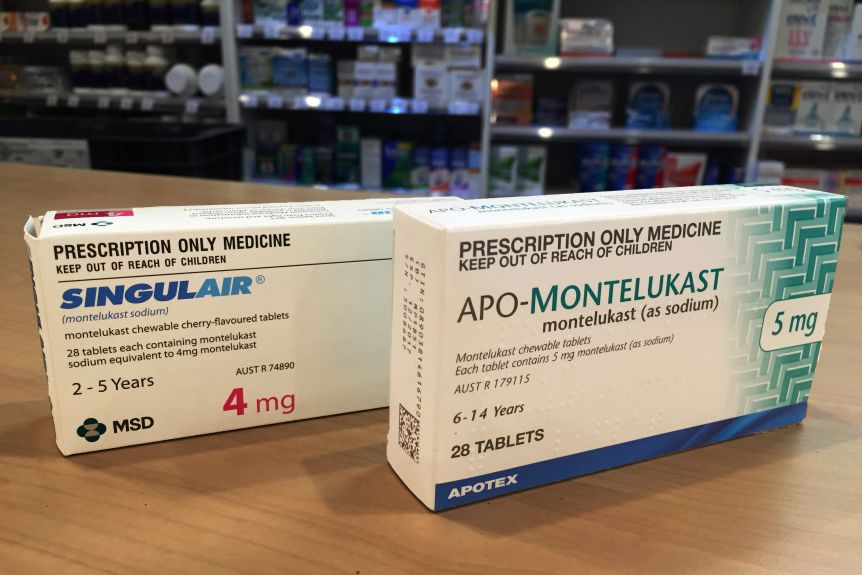
Singulair, also known as montelukast, is widely used to treat allergies and asthma. In March 2020, the Food and Drug Administration strengthened previous warnings for Singulair, requiring a black box warning. A black box warning is FDAs strongest warning, similar to the ones used for cigarettes. It warns about serious risks of a medication or treatment. The black box warning for Singulair describes psychiatric side effects such as aggression, depression, agitation, sleep disturbances, suicidal thoughts, and suicide. You can read Singulairs complete black box warninghere.
The FDA advises that Singulair should not be the first choice treatment for mild allergic rhinitis, which is another name for seasonal allergies and hay fever. That is the advice FDA gives when a treatment is not as safe or not as effective as an alternative treatment that is available. The FDA also recommends that healthcare providers weigh the potential risks and benefits before prescribing Singulair for asthma.1
Learning Objectives And Cme/disclosure Information
This activity is intended for healthcare providers delivering care to women and their families.
After completing this activity, the participant should be better able to:
1. List the FDA approved indications for use of montelukast in the treatment of asthma2. Discuss the reasons for the FDA requirement for a boxed warning and Medication Guide for montelukast
Estimated time to complete activity: 0.25 hours
You May Like: Eosinophilic Asthma Blood Test Results
What Should Health Care Professionals Do
Health care professionals should consider the risks and benefits of montelukast when deciding to prescribe or continue patients on the medicine. Counsel all patients receiving montelukast about mental health side effects, and advise them to stop the medicine and contact a health care professional immediately if they develop any symptoms included but not limited to those listed in the table above. Be aware that some patients have reported neuropsychiatric events after discontinuation of montelukast.
Only prescribe montelukast for allergic rhinitis in patients who have an inadequate response or intolerance to alternative therapies.
Recommendations For Safe Prescribing

One qualitative study in England found that family physicians prescribe new drugs more often than subspecialists, and obtain more information about new drugs from pharmaceutical representatives.26 Although it is unclear whether these trends are occurring in the United States, physicians must realize that all newly approved drugs pose a risk of unsuspected adverse events.27
The scope of family medicine makes it difficult for physicians to remain current with emerging information on all relevant drugs. As illustrated by the examples in this article, boxed warnings may be issued before definitive evidence substantiates a safety concern. In addition, boxed warnings alone cannot provide the context needed to individualize risks and benefits to each patient’s circumstances.
The STEPS model was originally developed as a tool to evaluate new drugs .28 Consideration of the five STEPS criteria helps physicians decide whether to prescribe a new medication or choose an older alternative. The STEPS model can also be used when deciding whether to prescribe a medication with a boxed warning. Do equally effective and safer alternatives exist? Does the potential benefit of the drug outweigh the safety concern?
Also Check: How To Give Yourself An Asthma Attack On Purpose
What Are The Side Effects Of Jak Inhibitors For Eczema
JAK inhibitors can alter your immune systems ability to react to pathogens, which can put you at risk for bacterial, fungal, or viral infections. Herpes viruses can reactivate, as well.
Another potential side effect is headaches, which people with migraine should be aware of. This could potentially make their migraine episodes worse.
Before starting treatment, patients should be screened and have their health history reviewed to see whether the medication is likely to be safe for them.
Often baseline lab work is required for certain medications, as well as for ongoing monitoring to ensure the treatment continues to be safe and well-tolerated.
Inflammation at a molecular level is very complex. There are different pathways that trigger and cause people with eczema to experience inflammation, irritation, and itching.
Corticosteroids and JAK inhibitors target that inflammation in different ways.
Steroid treatments are human-made medications that reduce inflammation in the skin. They interact to block the chemical necessary for inflammation, which can help reduce eczema symptoms.
JAK inhibitors can block more specific pathways that are involved with atopic dermatitis.
You and a healthcare professional should work together to determine whether to treat your eczema with a JAK inhibitor. The factors to consider include:
- how well your eczema is managed
- your quality of life
- cost of treatments
- potential risks versus benefits
Serious Reactions Of Nsaids
Serious reactions are those types of adverse reactions that can be life-threatening or cause severe damage to ones health. The serious reactions for NSAIDs, in general, follow:
These are side effects that you definitely do not want. But these arent even the biggest problems with NSAIDs. The biggest problem with NSAIDs is that they carry black-box warnings.
Also Check: What Does Asthma Do To Your Lungs
What Are Black Box Warnings
A black box warning often referred to as simply a boxed warning is the strongest warning issued by the FDA in the United States on drugs that carry specific health risks serious or life-threatening adverse effects.
When a black-box warning is issued, it informs healthcare providers and prescribers of serious adverse effects of specific drugs and enhances their clinical judgment. For example: when atypical antipsychotics were assigned a black box warning for use in patients with dementia prescription use of antipsychotics for this population declined thereafter.
The same is true for many other medicines, too. For instance, when the antidiabetic drug rosiglitazone was issued a black box warning, use of that medicine declined by almost three-quarters. Prescribers seek alternative medicine choices to reduce any potential risk to the affected population.
Here, we have put together a list of black-box warnings that both prescribers and pharmacists must know. Bear in mind that this is not intended to be a complete list of black box warnings.
Fda: Asthma Drug Singulair To Get ‘black Box’ Warning
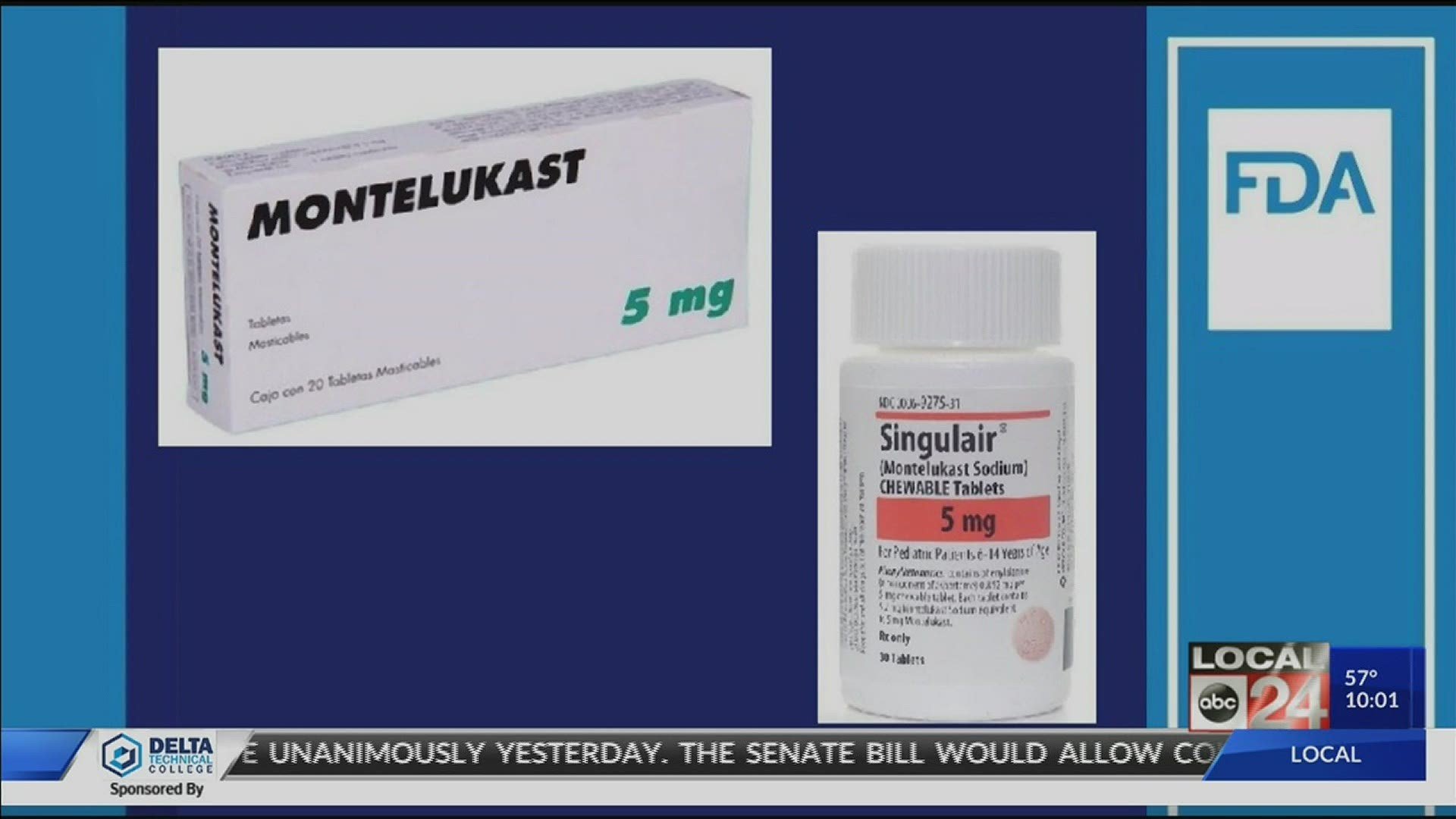
Asthma and allergy drug montelukastsold as a generic and under the brand name Singulairwill get a “boxed warning” over potential ties to neuropsychiatric effects, the U.S. Food and Drug Administration announced Wednesday.
The drug has long carried a warning that it has been linked with an increased risk for “agitation, depression, sleeping problems, and suicidal thoughts and actions,” the FDA said in a statement. The agency’s move Wednesday elevates that advisory to its most prominent boxed warning. The new warning advises health care providers to “avoid prescribing montelukast for patients with mild symptoms, particularly those with allergic rhinitis.” Added to the boxed warning, patients who are prescribed montelukast will also get a special Medication Guide outlining potential risks.
The first such warning added to montelukast labeling came in 2008 after reports of suicide and other serious psychiatric events were reported in users. The agency has since tracked and compiled data linking mental health issues with use of the drug, and a summary was presented at an FDA advisory committee meeting last year. Based on the committee’s assessment, “the FDA determined the risks of montelukast may outweigh the benefits in some patients, particularly when the symptoms of the disease are mild and can be adequately treated with alternative therapies,” the agency said.
Explore further
You May Like: How To Calm Down Asthma Without Inhaler
May Discourage People From Taking Medication
While black box warnings can decrease use of the medication for at-risk-populations, they might also discourage people who need medication from taking it, studies show.
Christine Y. Lu and colleagues at Harvard Pilgrim Health Care Institute published a 2014 study in the British Medical Journal. They found black box warnings decreased antidepressant use but increased the suicide attempt rate among young people.
The FDA, the media and physicians need to find better ways to work together to ensure that patients get the medication that they need, while still being protected from potential risks, Lu said in a Harvard press release.
Boxed Warnings And Physician Practice
Physician adherence to boxed warnings is voluntary no formal system exists to document appropriate patient selection, risk counseling, or drug monitoring. A large observational study of 51 outpatient practices in Boston, Mass., accessed electronic medical records to evaluate physician prescribing of drugs with boxed warnings.25 Of 324,548 prescriptions issued, 2,354 violated some aspect of a boxed warning . Nonadherence was more likely when prescribing for patients older than 75 years and for those taking multiple prescriptions. In this study, less than 1 percent of instances resulted in an adverse drug event.
Also Check: Can Essential Oils Help Asthma
Worried About A Warning Talk To Your Healthcare Provider
All medications come with potential risks and benefits. Ifyou have concerns about a medicine youre taking, dont delay bringing them upwith a healthcare professional who can help to put them in context or discussalternatives.
If your prescriber or pharmacist hasnt discussed them with you, contact either one of them for help answering any questions you might have, Dr. Lehmann says.
Update: Singulair And That Black Box Warning
- Reactions 0 reactions
Montelukast was approved by the FDA in 1998, 1 although I wasnt prescribed it until March of 2008. At the time my doctor said it was overhyped, although he agreed to let me try it. That spring was the first time in my life I made it through pollen season without feeling miserable due to sniffles, sneezes, and wheezes.
Little did I know at the time, in September of 2008 the Associated Press reported on a 15-year-old high school football player who started taking Singulair in 2007 due to an allergy to ragweed. Within weeks his parents noted he was feeling agitated and having trouble sleeping, and 17 days after starting the medicine he committed suicide. Singulair was blamed, and the case was reported to the FDA. 2
Don’t Miss: Can You Use Vicks If You Have Asthma
Links To Psychiatric Events Renewed
The issue of Singulair and Psychiatric events was brought up again on September 23, 2014, at a Pediatric Advisory Committee. Addressing the concerns of a parent who addressed the committee, the members reviewed the current data and determined that, despite the previously mentioned studies, there were many reports of patients or parents reporting psychiatric events, such as the ones mentioned in the black box warning listed above. While most psychiatric events reported were not serious, all were reversed after cessation of the product. 1, 7
To address these concerns, the members of the committee discussed the need to communicate with physicians to raise awareness of possible psychiatric side effects, to discuss possible side effects with patients/parents, to monitor patients for signs of psychiatric events, and to discontinue the medicine if any signs of psychiatric events are observed. 1, 7
How The Asthma Center Can Help
The board-certified asthma specialists at The Asthma Center are the Delaware Valleys leading experts on treating patients with asthma. With advanced in-office diagnostics, our asthma specialists are trained to correctly diagnosis and treat asthma symptoms and flares. Working with each patient individually, our allergists create a personalized asthma treatment plan to prevent and treat asthma. The board-certified asthma specialists at The Asthma Center treat patients in 9 convenient locations throughout the Delaware Valley.
Don’t Miss: What Happens If You Hit An Inhaler Without Asthma
Increased Use Of Black Box Warnings
In the last several years, the FDA has approved record numbers of new drugs. While drug approvals are up, so are their safety risks many of these lead to black box warnings.
In a 2017 JAMA study by Nicholas S. Downing and colleagues, researchers found that nearly a third of all drugs cleared by the FDA pose a safety risk.
It took the agency a little more than four years on average to take action against the drugs reviewed in the study.
What Safety Concern Is Fda Announcing
The U.S. Food and Drug Administration is strengthening existing warnings about serious behavior and mood-related changes with montelukast , which is a prescription medicine for asthma and allergy.
We are taking this action after a review of available information led us to reevaluate the benefits and risks of montelukast use. Montelukast prescribing information already includes warnings about mental health side effects, including suicidal thoughts or actions however, many health care professionals and patients/caregivers are not aware of the risk. We decided a stronger warning is needed after conducting an extensive review of available information and convening a panel of outside experts, and therefore determined that a Boxed Warning was appropriate.
Because of the risk of mental health side effects, the benefits of montelukast may not outweigh the risks in some patients, particularly when the symptoms of disease may be mild and adequately treated with other medicines. For allergic rhinitis, also known as hay fever, we have determined that montelukast should be reserved for those who are not treated effectively with or cannot tolerate other allergy medicines. For patients with asthma, we recommend that health care professionals consider the benefits and risks of mental health side effects before prescribing montelukast.
Recommended Reading: How To Use A Humidifier For Asthma
How Does A Medicine Get This Warning
FDA often identifies safety concerns with medications through clinical trial data or through reports of so-called adverse events submitted to the agency by consumers and healthcare professionals.
Its not always possible to know that those adverse eventsare directly caused by a medication, but if FDA identifies a serious concern,it can require a drug company to update its products labeling, restrict itsuse or, in rare cases, remove it from the market.
For example, several kinds of combination birth control pills carry a black box warning related to cardiovascular risks associated with them. The warning also strongly advises that women who use them not smoke, as cigarette smoking is known to increase the risk of those side effects.
Fda To Require Boxed Warning For Montelukast
Allergy & Asthma Networks voices were heard the U.S. Food and Drug Administration announced it is requiring a boxed warning for montelukast due to mental health, behavior and mood-related changes associated with the medication.
Allergy & Asthma Network was the only national patient organization to advocate for the black box warning, Tonya Winders, President and CEO of the Network, told Healthline.com. We have many reports of families impacted by the neuropsychiatric side effects of montelukast.
Montelukast prescribing information already includes warnings about mental health side effects, including suicidal thoughts and actions, however many healthcare professionals, patients and families are not aware of the risk.
The boxed warning is FDAs most prominent warning, added to the prescribing information for montelukast to describe these serious mental health side effects.
One family impacted by montelukast mental health side effects was the Teehee family of Tahlequah, Oklahoma.
It is important to recognize the warning signs and talk with your doctor about alternative treatments, Winders says.
If you or your child experiences mental health, behavior or mood-related changes while taking montelukast, FDA advises stopping the treatment and discussing the side effects with a doctor. These changes may include:
- Agitation or hostility
Don’t Miss: How To Reduce Asthma Problem
Fda Investigates Singulair And Suicides
In March of 2008, the FDA announced it was performing a review of the links between Singulair and psychological events . Shortly thereafter there were similar reports. 1
Prior to this time, the FDA only investigated psychiatric drugs for potential suicide risks. However, these reports opened the doors for them investigating other drugs that have the potential for crossing the blood brain barrier and having an effect on the mind, and this included asthma medicines. 2
During its first ten years on the market, Singulair became the best selling drug for Merck. In 2007 alone, sales were $3.4 billion. Merck reported that none of the 11,000 participants involved in initial studies of Singulair reported psychological events.
However, just to be on the safe side, and before any further studies were conducted, the FDA issued warnings on the products so that the public — asthmatics, parents, and doctors alike — were well aware of these reports. 3-4
Concerns About Singulair Black Box Warning Begin To Mount
This was thought to be good in that it would make the public aware of the reports, and hopefully inspire patient-physician or parent-physician discussions. However, most doctors ignored the warnings — including my own doctor at that time, and continued prescribing the medicine anyway, usually without issuing any warnings. The main reason was because they had already prescribed the medicine to so many asthmatics with good results.4
Another concern among the medical community about the warnings was that they might scare some asthmatics , thereby preventing some asthmatics from taking a medicine that might otherwise help them obtain improved allergy and asthma control.2
This is actually the reason I decided to write this update. My 14-year-old daughter, like me, has asthma and severe allergies. Now that my allergic asthma is relatively controlled thanks to both Advair and Singulair, I thought Singulair would help her control her allergies. So I discussed the idea of having my daughters pediatrician prescribe it for her. My wife was concerned due to the warnings.
My wife, my daughter, and I have had some nice discussions about Singulair. So far my daughter is managing without it. Still, there are mornings where shes sniffling and sneezing quite a bit, so theres a part of me that wonders if Singulair would be good for her, perhaps even improve her well-being. Perhaps she will have to end up making the decision on her own someday.
Read Also: How To Give Myself An Asthma Attack
